
Cellular Ag: Giving Your Plate a Biotech Reboot - Part 2
This is the second entry of a two-part blog.
It isn’t only animal cells that are being cultured for lab-based meats- several microbial sources are being developed as well. One particularly interesting source is fungal tissue; not mushrooms (aka, the fungal ‘fruit’), but filamentous structures called mycelia. Under favorable conditions, these organisms form dense networks of this tissue in order to absorb nutrients from their surroundings. Intriguingly, these tissues can take on a meat-like structure, making them a compelling meat alternative, especially given some of the challenges associated with building proper texture in cultured meats. Perhaps little known, a so-called ‘mycoprotein’ has already been on the market for decades: Quorn™️ is derived from mycelial growth of the fungal species Fusarium venanatum, which was originally selected for its high protein production in the 1960s from a screening of over 3,000 strains in the U.K. F. venanatum is grown in an engineered environment called an airlift fermenting bioreactor, allowing dense tissue growth, which can be harvested for Quorn production. Currently, Quorn’s parent company, Monde Nissin Corp. markets grounds, nuggets, and cutlets, but typically uses egg as a structural binding agent.
Start-ups such as Meati (Boulder, CO; est. 2018), MyForest Foods (Formerly Atlast; Albany, NY; est. 2019), and The Better Meat Company (West Sacramento; est. 2018) are also developing mycelial meats, with a strong emphasis on textured ‘whole-cuts’, boasting minimal food processing as a key innovation. Current products in development include chicken breast, steak, jerky, bacon, and pork chops (see Part 1, Table 1). To achieve the whole-cut meat-like texture, growth of mycelial tissue is induced in either a submerged or solid-state fermentation bioreactor, depending on the desired shape, density, and thickness of mycelial slabs (Figure 1). MyForest Foods purports that their total ‘seed to harvest’ grow time is just 10 days, and Meati claims production will soon ramp up to approximately 4,500 cattle-equivalent of mass per 24 hours with outputs achieving greater than 60% protein content by weight. Since mycelial tissue is intrinsically flavorless, relatively similar bioreactor processing protocols can be used across a variety of products, which can then be differentially flavored. Quick growth times plus optimized fermentation procedures already make mycelial meat cuts price-competitive with real meat, giving ‘myco-meats’ a distinct advantage over cell-cultured products. The Better Meat Co. is taking a compromise approach to meat competition: while some of its products are fully meatless, they also have a line of ‘enhanced’ meats in development that contain approximately one-third mycelial protein (their proprietary formulation called Rhiza). These blended meats offer superior nutritional profiles (e.g., reduced cholesterol and full amino acid spectrum) compared to meat alone, lower product cost, and may be more attractive to customers who are skeptical of alternative proteins as compared to pure mycelial products.

Figure 1. Conceptual diagram of mycoprotein production from fermentation of filamentous fungi. Mycelia can be cultivated using several bioreactor types including airlift, submerged, and solid-state (pictured), and fed with simple, starch-based media. Black, bolded text describes key steps or elements of production. Green, italicized text proposes aspects of current design which may increase economic efficiency or reduce environmental impact/energy demand.
Some cellular agriculture start-ups promise to take dairy production off the farm as well, and products are likely to arrive at a global commercial scale much faster than meats since tissue structural integrity is not a technological hurdle. In fact, components of dairy-milk based products have been produced in a lab for decades: Chymosin, for example, originally derived from the stomach lining of ruminant calves, catalyzes coagulation of milk proteins (casein and whey), and is useful for cheese production. However, the vast majority of cheese produced today uses fermentation-produced chymosin (FPCs) from recombinant bacteria or fungi. Biotech start-ups such as Perfect Day are taking this a step further by producing milk proteins via recombinant yeast fermentation (Figure 2). Isolated protein can then be added to water as well as plant-derived fats and sugars in specified ratios to produce consumer favorites such as ice cream and cheese. Perfect Day also purports their fermentation-derived dairy products will have longer shelf life than conventional milk. Through a partnership with Brave Robot, Perfect Day is already producing real milk protein, plant-based ice creams commercially.
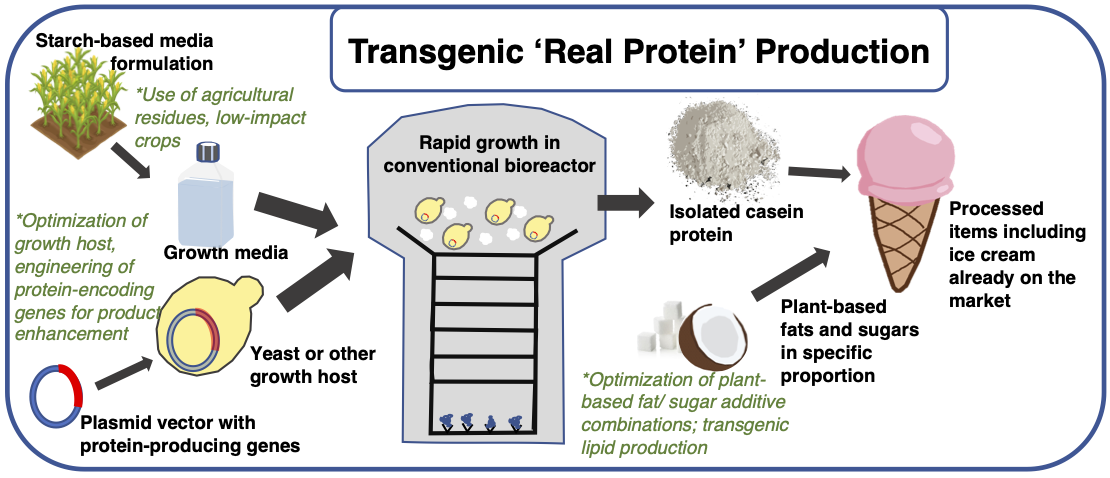
Figure 2. Conceptual diagram of transgenic animal protein production. Recombinant plasmids carrying a variety of protein-encoding genes such as whey, casein (pictured), or egg white proteins can be replicated in competent yeast or bacteria, and purified product subsequently isolated. Fermentation of whole organisms drastically lowers culturing complexity compared to differentiated mammalian cells. Black, bolded text describes key steps or elements of production. Green italicized text proposes aspects of current design which may increase economic efficiency, quality or quantity of product, or reduce environmental impact/energy demand.
Another emerging lab-based commodity likely close to market is egg white protein (EWP). Biotech startup The Every Company is producing a variety of EWPs (e.g., ovoalbumin, ovotransferrin, ovomucoid) via fermentation by recombinant microbes, and blending them to produce a product suitable for baking or scrambling that is also nutritionally identical to egg whites. Individualized production of different EWPs also offers a distinct culinary advantage over the chicken-based version: Identification of how EWPs in varying combinations and proportions contribute to desired properties such as binding, foaming, and emulsifying allows the development of products tailor-made for specific culinary applications.
The large number of start-ups in the cellular agriculture space (see Part 1, Table 1) suggests food supply chains may shift dramatically over the next decade. Given the technical and cost-efficiency challenges associated with whole-cut, animal meat production at this time, it is much more likely that grounds, nuggets, and patties, as well as dairy, EWPs, and whole-cut ‘myco-meats’ will appear on the market en masse first. There are several health and safety benefits that may boost their popularity, including:
- Production of proteins away from the animal means a reduced risk of foodborne illness and negates the need for widespread hormone and antibiotic use
- Nutritional aspects such as protein to fat ratio, as well as the inclusion of micronutrients may be beneficial for fighting malnutrition in some regions, and obesity in others
- With the animal largely taken out of the equation, dense factory farming would likely decline, addressing concerns for animal welfare and waterway pollution from manure runoff.
Switching from animal- to cell culture-based production would drastically decrease land use requirements, as well as agricultural production of methane and nitrous oxide, two potent greenhouse gases. Impacts on net emissions of carbon dioxide with cell-cultured meats are less clear, and dependent on how production systems are implemented and managed (e.g., whether waste products are reused or repurposed for energy offsets). Limited available life cycle assessments show lab-grown meat may have marginally lower energy requirements for production compared to beef, but considerably higher compared to chicken or pork. Contrastingly, mycoprotein appears to have similar overall energy requirements for production as chicken and pork. Aspects of current mycelial meat production such as the use of glucose as a fermentation feedstock likely contribute substantially to overall energy demands, and innovation such as lower-impact growth media could help to make this alt-meat more environmentally favorable. Net environmental impacts will likely be an important consideration as products begin coming to market.
There are of course, some downsides associated with cellular agriculture that may ultimately undermine sector growth- namely public perception and consumer cost. For example, across a large number of polls, opinion of ‘farmless’ meat and dairy products varies widely, and objection is commonly raised in relation to their perceived ‘unnaturalness’. Ultimately, consumers will likely judge these products based on price point, availability (e.g., are they in fast food chains?), and overall taste comparison. Another issue raised is job loss in the agricultural sector. However, a steep decline in land needed for livestock production brings opportunities for alternative crop production or soil carbon banking. Organizations such as The Transfarmation Project and Refarm’d are assisting farmers in accessing financial and technical resources to phase out livestock production while phasing in high-value crops like hemp. Additionally, there may be new, higher profit margin opportunities emerging in animal breeding for cell culture sourcing. Cellular agricultural products will begin to hit the market over the next several years; public consumption and engagement with this budding industry will be fascinating to see.
References
Altamirano, C., Berrios, J., Vergara, M., and Becerra, S. (2013). Advances in improving mammalian cells metabolism for recombinant protein production. Electronic Journal of Biotechnology 16, 10-10.
Denny, A., Aisbitt, B., and Lunn, J. (2008). Mycoprotein and health. Nutrition bulletin 33, 298-310.
Mattick, C. S. (2018). Cellular agriculture: The coming revolution in food production. Bulletin of the Atomic Scientists 74, 32-35.
Mendly-Zambo, Z., Powell, L. J., and Newman, L. L. (2021). Dairy 3.0: cellular agriculture and the future of milk. Food, Culture & Society 24, 675-693.
Mistry, V. (2012). Chymosin in cheese making. Food biochemistry and food processing 52, 1170-1176.
Pandya, R. (2014). Milk without the moo. New Scientist 222, 28-29.
Post, M. J. (2012). Cultured meat from stem cells: Challenges and prospects. Meat science 92, 297-301.
Rischer, H., Szilvay, G. R., and Oksman-Caldentey, K.-M. (2020). Cellular agriculture—industrial biotechnology for food and materials. Current opinion in biotechnology 61, 128-134.
Rojas-Downing, M. M., Nejadhashemi, A. P., Harrigan, T., and Woznicki, S. A. (2017). Climate change and livestock: Impacts, adaptation, and mitigation. Climate Risk Management 16, 145-163.
Smetana, S., Profeta, A., Voigt, R., Kircher, C., and Heinz, V. (2021). Meat substitution in burgers: nutritional scoring, sensorial testing, and Life Cycle Assessment. Future Foods 4, 100042.
Southey, F. (2021). Cracking the ‘world’s first’ animal-free egg white through fermentation. In "foodnavigator.com", Vol. 2021. William Reed Business Media Ltd.
Stephens, N., Di Silvio, L., Dunsford, I., Ellis, M., Glencross, A., and Sexton, A. (2018). Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends in food science & technology 78, 155-166.
Watson, E. (2021). Meat from mycelium: Fungi fueled startup Meati Foods raises $50m to make Holy Grail of alt meat: whole cuts. Vol. 2021. William Reed Business Media Ltd.
Whittaker, J. A., Johnson, R. I., Finnigan, T. J. A., Avery, S. V., and Dyer, P. S. (2020). The Biotechnology of Quorn Mycoprotein: Past, Present and Future Challenges. In "Grand Challenges in Fungal Biotechnology" (H. Nevalainen, ed.), pp. 59-79. Springer International Publishing, Cham.
